
INTERCEPT innovative technology represents the most safe and reliable system to remove virus, bacteria, protozoa and leukocyte contamination in blood components. The efficacy is proven on a wide range of pathogens including the emerging ones.
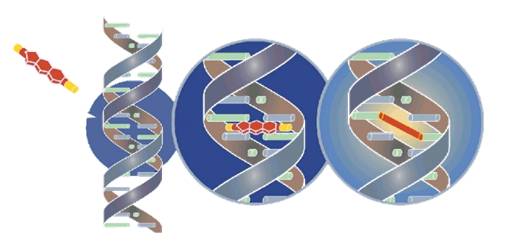
INTERCEPT technology uses amotosalen HCl, a well characterized photoactive compound that specifically targets DNA and RNA , and UVA illumination to irreversibly cross-link nucleic acids. In doing so, INTERCEPT blocks replication of viruses, bacteria, and parasites, making them inactive.
In addition, the number of T-cells is reduced to a level that reduces the risk of transfusion-associated graft-versus-host disease (GvHD).

Bacterial contamination of platelet products is the most significant infectious risk in transfusion today. About 1 in 1,500 platelet units is estimated to contain bacteria, even after culture detection, translating to a 1 in 250 per patient risk.
The INTERCEPT Blood System for Platelets achieves effective inactivation for a breadth of bacteria with varying growth rates.
The safety and efficacy of INTERCEPT Blood System processed platelets has been supported by hemovigilance programs and several clinical trials.
Currently INTERCEPT platelet and plasma inactivation is in routine use in more than 100 blood banks and 20 countries.
For further information, please visit Intercept website
